Sunlight is integral to many chemical processes that occur every day. Photosynthesis, for example, converts light energy into chemical energy, providing much of the energy required for life on earth.
In previous attempts in laboratory settings to study chemical processes powered by sunlight, researchers have relied mostly on pulsed-laser experiments. However, the light created by lasers differ in important ways from sunlight, particularly in their strength, their frequency and their duration of incidence.
In order to better understand how biological chemical processes work under natural sunlight, Professor Paul Brumer and postdoctoral fellow Dr. Chern Chuang, in work funded by the U.S. Air Force Office of Scientific Research, have created a computational model that properly mimics aspects of nature.
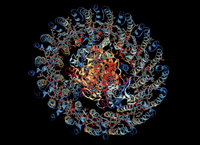
They were able to simulate the photocycle of the light-harvesting complex 1 (LH1) and the associated reaction center in purple bacteria driven by natural incoherent light (pictured to the right). Brumer uses an analogy to compare incoherent sunlight with pulsed-laser light and explains why we should be using the former to study biological processes.
“Imagine trying to study what happens to a goalie in hockey under two different scenarios. If you used a professional hockey player to hit the puck toward the goal, then every shot would be nearly perfectly executed. Struck on the same spot with the same speed and intensity every time. This is the analogue of photons from a pulsed laser incident on a molecule.”
“But now imagine getting a wide variety of people of differing strengths and expertise to hit the puck, maybe multiple people striking the puck at the same time. The goalie would be dealing with randomly incident pucks. This is the analogue of photons from incoherent solar light incident on a molecule. The response of the molecule in these two cases is dramatically different.”
The future of this work is exciting and Brumer sees the potential application not only in biological research but also in vision and solar cell research.
“It is very promising that both theory and computational tools have now advanced to the point that we can treat complex biological processes, such as energy transfer in this bacterial system, powered by natural light,” says Brumer.
“The results both advance our understanding of these complex processes and allow input into a wide variety of applications.”